
This is exactly what we are doing when we calculate the relative molecular mass of a compound: Mass(water molecule) = mass(oxygen atom) + 2 × mass(hydrogen atom) We could write a mathematical expression to find the total mass of all the atoms in a water molecule: If an oxygen atom has a mass of 16 and a hydrogen atom has a mass of 1, then the mass of all three atoms in the water molecule is 16 + 1 + 1 = 18 each diagonal line ( \ and / ) represents a chemical bond between an oxygen atom and a hydrogen atom.In the diagram of a water molecule above: If each ball in the box represents an atom making up a water molecule, H 2O, then the diagram below shows a box containing a molecule of water: Mass(total) = mass(red ball) + 2 × mass(black ball)Ĭhemists often think of atoms as really tiny balls, and refer to this as the particle theory of matter. We could write a mathematical expression to find the total mass of the balls in the box: If a red ball has a mass of 16 g and a black ball has a mass of 1 g, then the mass of all three balls in the box is 16 + 1 + 1 = 18 g The diagram above shows a box containing 3 balls:
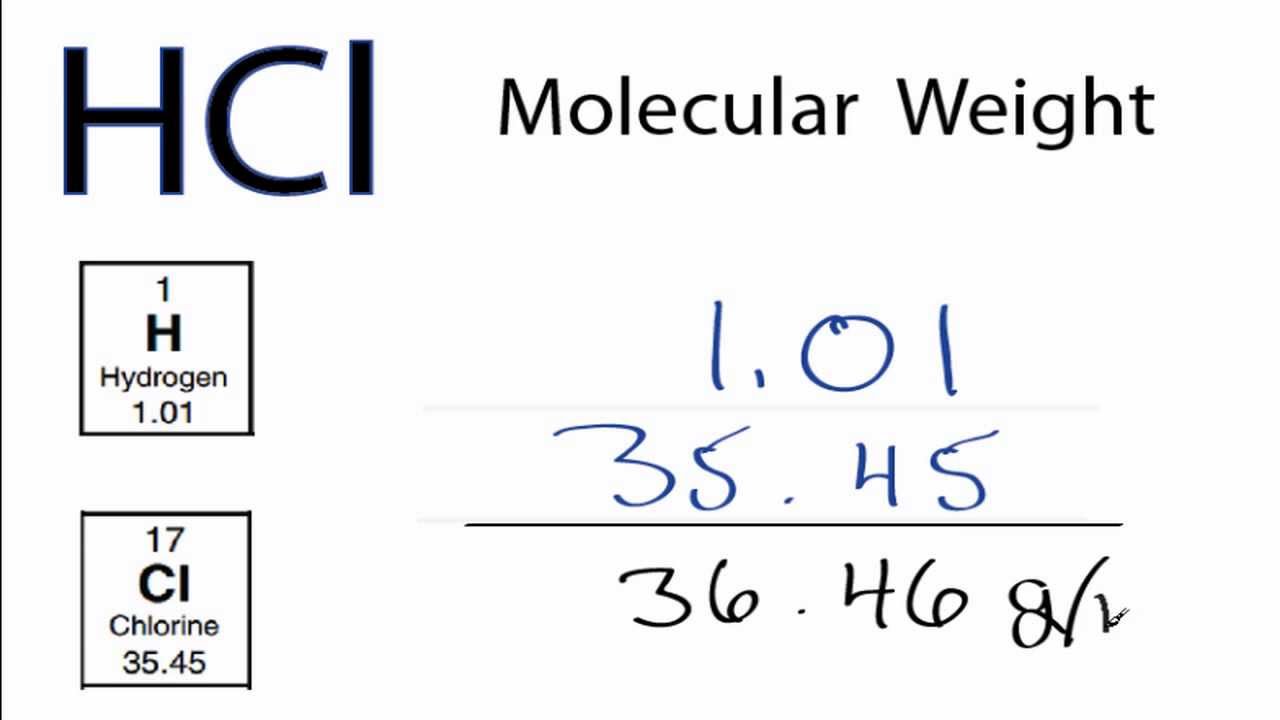
#Molar mass of hydrogen free
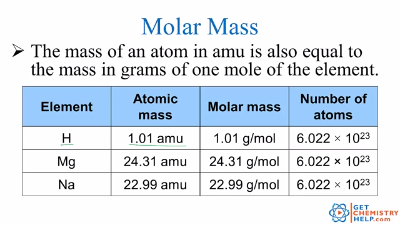
No ads = no money for us = no free stuff for you! Step 4: Substitute the values for the relative atomic mass (atomic weight) of each element into the equation and solve.Step 3: Use the Periodic Table to find the relative atomic mass (atomic weight) of each element.Step 2: Write a mathematical expression to calculate the total mass of all the elements in the compound (the relative molecular mass of the compound).Step 1: Use the chemical formula to determine how many atoms of each element are present in the compound.⚛ There are 4 steps to calculating the relative molecular mass of a molecule or compound given its chemical formula: ⚛ Relative molecular mass can be treated as a dimensionless quantity, that is, a quantity that has no units. formula of a molecule (or molecular compound).⚛ In practice, the relative molecular mass of a compound, M r, is the sum of the relative atomic masses ( atomic weights) of the atomic species as given in the chemical formula. ⚛ Relative molecular mass of a compound ( M r) is defined as the "ratio of the mass of a molecule to the unified atomic mass unit" (2), or, the mass of a formula unit of the compound relative to the mass of a carbon-12 atom taken as exactly 12. ⚛ Relative molecular mass is usually given the symbol M r. ⚛ Relative molecular mass is also known as:


 0 kommentar(er)
0 kommentar(er)
